| Startseite |
| Impressum |
| Datenschutz |
| arznei-telegramm 2008; 39: 69 | |
| CHILDREN: NEUROLEPTIC USE RISING
|
|
A leading employee of Eli Lilly (USA) advised sales representatives in an e-mail that became public in March of this year to promote not only atomoxetine (STRATTERA) for juveniles with attention-deficit hyperactivity disorder (ADHS) with paediatricians and child or adolescent psychiatrists, but also the "atypical" neuroleptic olanzapine (ZYPREXA, generics), which is not licensed for this age-group (1). Such marketing strategies and the extension of psychiatric diagnoses such as bipolar disorder to children might be reasons why children and adolescents have increasingly been receiving antipsychotics for years (2-5). In Europe there have so far been hardly any figures. A recent Netherlands study (6) with data on approx. 500,000 insured persons now shows a marked rise: from 1997 to 2005 antipsychotic use among under-19 year olds has more than doubled from 30 to 68 per 10,000 adolescents per year. While the use of classical neuroleptics remained almost constant, the use of "atypicals" has increased nine-fold. Especially in five- to nine-year olds, "atypical" agents are prescribed particularly often at first time treatment. In this age group, the duration of use of neuroleptics is also the longest with a median of 5.1 years (6). The data from about 400 British general practices also contain about twice as many neuroleptics among 18-year olds in 2005 as in 1992. The rise affects mainly children and is based particularly on off-label use (7). According to data from the Scientific Institute (WIdO) of the AOK [Statutory Sickness Fund], prescriptions of olanzapine and risperidone (RISPERDAL, generics) for 5- to 15-year olds as well as for 15- to 20-year olds are increasing in Germany too (see table) (8). Of all "atypicals" only risperidone is licensed in Germany for children and adolescents and only for behavioural disorders in conjunction with intellectual disability. Despite inadequate data on efficacy and safety and the lack of licensing of the other "atypicals" the inhibition for prescribing these neuroleptics off-label in children and adolescents is obviously vanishing. This is indicated by the substantial number of off-label prescriptions of olanzapine and quetiapine (SEROQUEL) for 15- to 20-year olds. In contrast to the claimed harmlessness and supposed good tolerability, however, adverse events of "atypical" neuroleptics, for example olanzapine, such as sedation or extrapyramidal motor symptoms occur more frequently and more markedly in children than in adults (9-13). This also applies for weight gain with the risk of severe metabolic problems (1,9,13,14). For instance, in a German study, 12- to 19-year olds gained an average of 5 kg after taking olanzapine for only six weeks and an average of 12 kg after a further 18 weeks (15). The solution recommended in a study commissioned by Eli Lilly was addition of the biguanide antidiabetic drug metformin (GLUCOPHAGE, generics) to reduce the drug-induced weight gain again (not a licensed indication) (16). As with the other "atypicals" not licensed for children, there is not only no proof of efficacy for olanzapine but data are also lacking on the long-term safety for the still-developing child's body (9). Table: Ambulant prescriptions of a few "atypical" neuroleptics for children (Germany, Statutory Sickness Fund) in 2001 and 2006 in defined daily doses (DDD) per 1,000 insured persons, classified by age groups (8) 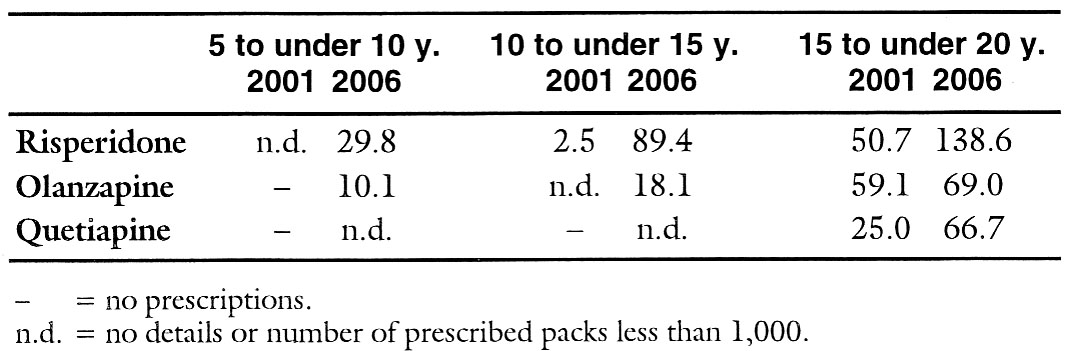
|
| (R = randomized study) | |
1 | New York Times of 15th March 2008 | |
2 | FINDLING, R.L. et al.: J. Clin. Psychiatry 2005; 66 (Suppl.7): 29-40 | |
3 | ||
4 | DOMINO, M.E., SWARTZ, M.S.: Psychiatric Services 2008; 59: 507-14 | |
5 | AACAP Official Action: J. Am. Acad. Child Adolesc. Psychiatry 2007; 46: 107-25 | |
6 | KALVERDIJK, L.J. et al.: Psychiatric Services 2008; 59: 554-60 | |
7 | ||
8 | Scientific Institute of the AOK [Statutory Sickness Fund], analysis of 30th May 2008 | |
9 | CURTIS, L.H. et al.: Arch. Pediatr. Adolesc. Med. 2005; 159: 362-6 | |
| R | 10 | |
11 | ||
12 | ||
13 | ||
14 | JERRELL, J.M., McINTYRE, R.S.: Hum. Psychopharmacol. 2008; 23: 283-90 | |
15 | DITTMANN, R.W. et al.: J. Child Adolesc. Psychopharmacol. 2008; 18: 54-69 | |
| R | 16 |
| © arznei-telegramm 6/08 | ||
|
| ||