IN BRIEF
Blockage of the adrenaline auto-injector EMERADE
At the end of June, Bausch + Lomb informs about blockage of the EMERADE adrenaline auto-injector which occured "in very rare cases" during stability checks (1). The auto-injector is authorised for the emergency treatment of severe acute allergic reactions. If there is no clinical improvement after the first injection, a second pen can be used to inject a further dose 5 to 15 minutes after the first. The Summary of Product Characteristics (SPC) recommends prescribing patients two auto-injectors that they should carry with them at all times (2). Due to the identified problem it is now "absolutely essential" for patients to carry two pens with them (1). Given that a blockage cannot be ruled out and it may be necessary to administer a second injection, we are now faced with the question whether it would be necessary to prescribe three pens. However, in light of the high costs of the auto-injectors, the providers should be expected to guarantee that these will trigger reliably. Problems with adrenaline auto-injectors are not new. In the past few years, all three competitors have recalled injectors at the patient level for possible malfunctions: Lincoln Medical in 2012 ANAPEN and ANAPEN JUNIOR (a-t 2012; 43: 62), ALK in 2013 several batches of JEXT (3) and Meda in 2017 several batches of FASTJEKT and FASTJEKT JUNIOR (a t 2017; 48: 40).* The costs for two injectors that have a maximal shelf-life of two years and for which the shelf-life has been reduced considerably in some cases in the past are at least EUR 149 calculated over a two-year period (see table), which we think is much too high. The delay between production and dispensing in the pharmacy, which decreases the potential period of use, is not even taken into account,-Ed.
| 1 | Bausch + Lomb: Dear Doctor Letter, as at 25 June 2018; http://www.a-turl.de/?k=tauc |
| 2 | Bausch + Lomb: SPC EMERADE, as at Dec. 2016 |
| 3 | Alk-Abelló: Dear Doctor Letter, as at 11 Nov. 2013; http://www.a-turl.de/?k=esit |
| 4 | BfArM: Letter dated 20 July 2018 |
| * | BfArM overviews 24 reports of events from Germany in which no injection was administered in an acute case due to a malfunction (4). |
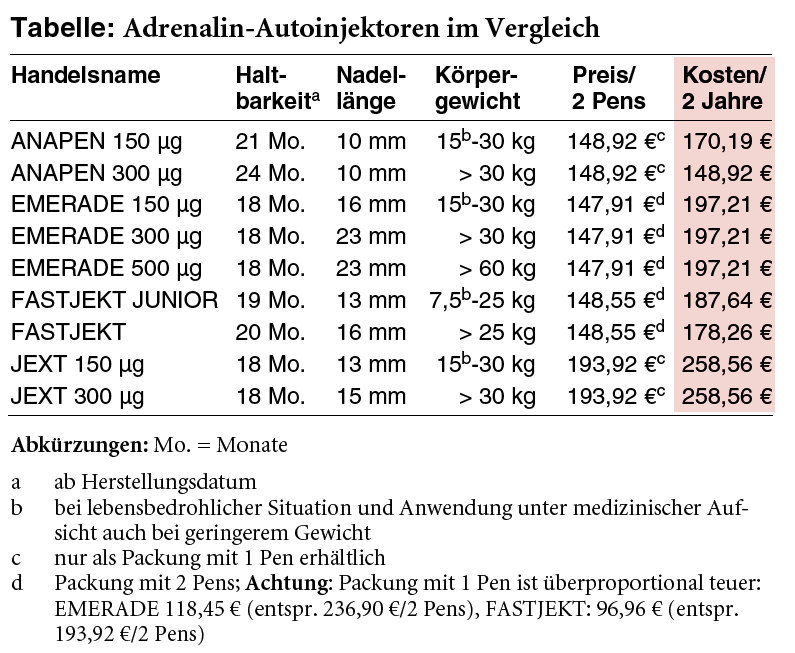
| a | = from the date of manufacture |
| b | = in life-threatening situations and use under medical supervision even in lower weights |
| c | = only available as a package with 1 pen |
| d | = package with 2 pens; warning: the package with 1 pen is disproportionately expensive: EMERADE EUR 118.45 (corresponds to EUR 236.90 for 2 pens), FASTJEKT: EUR 96.96 (corresponds to EUR 193.92 for 2 pens) |
© arznei-telegramm, August 2018, protected by copyright laws.
Autor: Redaktion arznei-telegramm - Wer wir sind und wie wir arbeiten
Diese Publikation ist urheberrechtlich geschützt. Vervielfältigung sowie Einspeicherung und Verarbeitung in elektronischen Systemen ist nur mit Genehmigung des arznei-telegramm® gestattet.
